DOI: ten.1039/C5TX00123D (Paper) Toxicol. Res., 2015, four, 1297-1307
Development and use of in vitro alternatives to animal testing by the pharmaceutical industry 1980–2013
Received 23rd April 2015 , Accepted 27th June 2015
Showtime published on 30th June 2015
Abstruse
We examined the employ of in vitro (including in silico) techniques in preclinical safety testing by the pharmaceutical industry between 1980 and 2013 to decide patterns, drivers and challenges in uptake. Data were collected via a survey sent to the Clan of the British Pharmaceutical Industry (ABPI) member companies from the Nonclinical and Biological Discovery Skilful Network (NaBDEN) requesting the number of compounds screened using in vitro and in silico tests at v-yr intervals between 1980 and 2005 then yearly from 2008 onwards. A utility score from 1 (poor) to v (excellent) for each assay was also requested. Four pharmaceutical companies and 3 contract research organisations (CROs) responded to the survey, providing >895![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 information points beyond all years and all assays. Overall, there was a steady increase in the use of in vitro tests by the pharmaceutical industry between 1980 and 2013; indeed >20% of all in vitro tests reported were conducted in the last yr of the survey window (2013) and >seventy% of all in vitro tests reported were conducted since 2010. Use of in vitro tests peaked at >190
000 information points beyond all years and all assays. Overall, there was a steady increase in the use of in vitro tests by the pharmaceutical industry between 1980 and 2013; indeed >20% of all in vitro tests reported were conducted in the last yr of the survey window (2013) and >seventy% of all in vitro tests reported were conducted since 2010. Use of in vitro tests peaked at >190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tests per annum in 2012; >99% of this usage was in the three main areas reported of ADME, condom pharmacology and genotoxicity. Trends and pace changes in uptake were virtually notable in the iii main areas of ADME, safety pharmacology and genotoxicity and may be explained by the timing of adoption of the relevant International Committee on Harmonisation (ICH) guidelines. Trends in uptake may as well exist explained by perceptions of utility where scores varied from poor (Middle Irritation – flourescein leakage) to splendid (Genotoxicity – Ames and Peel irritation – EpiSkin/Epiderm). In summary, the data show a large increase and a standing upwards trend in development and adoption of in vitro alternatives to animal testing in pharmaceutical drug development providing new opportunities to improve success rates coupled with a potent delivery to the 3Rs.
000 tests per annum in 2012; >99% of this usage was in the three main areas reported of ADME, condom pharmacology and genotoxicity. Trends and pace changes in uptake were virtually notable in the iii main areas of ADME, safety pharmacology and genotoxicity and may be explained by the timing of adoption of the relevant International Committee on Harmonisation (ICH) guidelines. Trends in uptake may as well exist explained by perceptions of utility where scores varied from poor (Middle Irritation – flourescein leakage) to splendid (Genotoxicity – Ames and Peel irritation – EpiSkin/Epiderm). In summary, the data show a large increase and a standing upwards trend in development and adoption of in vitro alternatives to animal testing in pharmaceutical drug development providing new opportunities to improve success rates coupled with a potent delivery to the 3Rs.
Introduction
Preclinical safety testing of new drug candidates is a crucial step in pharmaceutical drug development and depends on a sequential serial of in silico, in vitro and in vivo tests before assistants to humans. Currently, in vivo testing is a vital part of safe assessment, and is a regulatory requirement earlier a drug tin progress into clinical trials. 1,2 However, in recent years, many in vitro assays have been adult and validated for early on stage screening aimed at filtering out molecules with a higher potential for toxicity and in some cases replacing or reducing the utilise of certain in vivo tests.
The pharmaceutical industry's interest in developing new in vitro assays has arisen from the need to support the early on identification of promising drug candidates merely also through legislation requiring adherence to the 3Rs, a fix of principles that outlines the replacement, reduction and refinement of the utilize of animals in research. 3
These 3Rs have long been embedded in the United kingdom of great britain and northern ireland Animals (Scientific Procedures) Act (ASPA) 1986, 4 recently revised to transpose European Directive 2010/63/Eu 5 into new legislation. The European Medicine Agency's (EMA) paper on replacement of fauna studies by in vitro models 6 provides information on the conditions and strategy for regulatory acceptance of 3R alternative methods. Additionally, since its establishment in 2004, the UK National Centre for the 3Rs (NC3Rs) has played an of import role in promoting awareness of the 3Rs and in leading and driving the discovery, utilize and commercialisation of new non-animal technologies and alternative techniques. seven The United kingdom government has as well demonstrated its back up for the 3Rs, committing in 2010 to work towards reducing the use of animals in research, and recently publishing a Delivery Program 8 that details current and future initiatives to reduce the use of animals in research. Additionally, organisations that support regulatory validation of alternatives methods have been established, such equally the European Matrimony Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM). This has resulted in validation of a number of in vitro assays such as the bovine corneal opacity and permeability (BCOP) examination and Cytosensor microphysiometer exam that are used in heart irritation testing 9 and are now part of international regulatory guidelines. Finally, new technological and scientific advances, such as powerful computational models, and 'omics' technologies, take facilitated the development of many new in vitro assays.
The pharmaceutical industry has shown a strong delivery to the 3Rs principles, x–13 as demonstrated past their strong links with the NC3Rs 5 and their contributions to the Concordat on Openness on Animal Inquiry in the Britain. 11 The industry is increasingly working to replace, reduce and refine the use of animals in drug development and especially in toxicology testing; 12–14 legislative and regulatory changes, coupled with technological and scientific developments have provided opportunities to back up the adoption of in vitro and in silico alternatives to in vivo testing. Here, we have examined how the use of in vitro techniques within the pharmaceutical industry has evolved from 1980 to 2013 and report on where the uptake of in vitro and in silico techniques have been most notable both within pharmaceutical companies and CROs.
Methods
Collection of information
Excel spreadsheet was designed and adult by the Association of The British Pharmaceutical Manufacture (ABPI) Nonclinical and Biological Discovery Expert Network Group (NaBDEN) 15 to capture information on the historical use of in vitro assays (including in silico assays) in pharmaceutical R&D. The spreadsheet listed in vitro and in silico assays within the field of preclinical safety testing (Table 1) and provided fields to capture number of compounds put through each of the assays at v-year intervals between 1980 and 2005 and each yr from 2008 onwards. For each assay, the following information was requested: (i) the number of compounds screened per year using the assay and (two) a utility score of 0–5 based on the percieved value of the assay to the contributing company (Table 2). In addition to providing information requested, participants were invited to provide additional examples of in vitro assays used in their organisations.
Tabular array i Fields and tests listed in the survey spreadsheet
| Field of study/field | Exam/study type |
| DEREK: Deductive estimation of risk from existing cognition; MNU: Micronucleus analysis; Attain: Registration, evaluation, authorisation and restriction of chemicals; BCOP: Bovine corneal opacity and permeability; HET CAM: Hen's Egg test-chorioallantoic membrane; ECVAM: European centre for the validation of alternative methods; FP6: Framework programme 6; FP7: Framework programme 7; EURL: European Marriage Reference Laboratory; SAR: Structure activity relationship; ADRs: Adverse drug reactions; PK: Pharmacokinetics; NRU: Neutral red uptake. |
| Genotoxicity | COM jail cell transfection assays |
| DEREK + other in silico projects |
| Ames Ii, MNU, Greenscreen and Blueish screen assays |
| Impurity testing in silico for REACH |
| Mouse lymphoma assay |
| In vitro micronucleus examination |
| Ames |
| In vitro chromosome abnormality test |
| In silico Ames prediction GWS |
| SOS UMU |
| Prophylactic pharmacology | Electrophysiology testing |
| Cardiovascular |
| Human recombinant activity |
| In silico prediction for off target panel screen |
| Radioligand binding & enzyme – off target panel screen |
| Safe screen – cellular/functional |
| Skin irritation | Irritation (OECD 439) |
| EpiSkin (VRM) |
| SkinEthic RHE |
| EpiDerm SIT |
| Skin corrosion (OECD 430/431/435 | OCED 431 In Vitro Skin Corrosion (EpiDerm) |
| Dermal absorption | Pare absorption: in vitro/radio-analysis/LC-MS/MS |
| Heart irritation | BCOP |
| Isolated chick eye |
| Cytosensor microphysiometer |
| Fluorescein leakage |
| HET-CAM (ECVAM Validation) |
| Human being corneal epithelium |
| Development/reprotox/endocrine | Differential gene expression, mechanisms of action |
| FP6 and FP7 repro and neurotoxicity |
| ReproTECT |
| Endocrine disruptors – battery testing in vitro |
| Carcinogenicity Studies | FDA/Regulators encouraging use of bioassays |
| CarcinoGenomics FP6 |
| EURL ECVAM recommendation |
| Immunotoxicity | Human and mouse bogus lymph nodes |
| ADME | SAR before in vitro testing and in vivo testing for metabolic stability |
| CaCO ii efflux assays and transporter assays for prediction of ADRs |
| PK backdrop (assay beneath) |
| - CYP inhibition/induction |
| - Poly peptide binding in vitro |
| In vitro metabolic stability |
| Mechanistic studies |
| In vitro reactive metabolites |
| LINK programme – CYP expression |
| Microfluidics/culex |
| PD samples quantification (plasma, brain, tumours) |
| Phototoxicity | 3T3 NRU |
Table two Utility scores of 0 to 5 with explanation were requested as feedback from respondents on the utility of each in vitro test and were used to assist in interpretation of historical trends in the utilise of the in vitro assays
| Score | Reason |
| 0 | Do not believe in assay |
| 0 | Did non exist at this fourth dimension |
| 0 | Non engaged in R&D requiring this |
| 0 | Non valued/does not work |
| ane | Not predictive |
| 2 | Non very predictive |
| 3 | Reasonable |
| four | Skilful but not exhaustive |
| five | Excellent |
The survey was distributed to ABPI NaBDEN member companies with guidance together with a guarantee of anonimity for participating companies.
Data handling
Completed surveys were anonimised and collated by the number of reported tests by year and utility scores values.
Information returned on some tests were excluded from analysis, either considering they are not in vitro tests or because information from both in vivo/in vitro tests were combined and could not be separated in a meaningful way. These are listed in Table 3.
Table 3 Data were returned on the post-obit tests but were excluded from the study
| Discipline/field | Tests/study/project type |
| Safety pharmacology | Zebrafish |
| Developmental/reproductive/endocrinology toxicology | Fingerprint biomarkers (inhibin B) |
| Carcinogenicity | Transgenic onco mouse |
| ADME | In vivo bioavailability |
| In vivo absorption |
| In vivo reactive metabolites |
| Metabolic identification (in vivo/in vitro) |
Where respondents provided data in absolute values, e.g. seven, 30, 699, 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 098 these were used in the analysis. Where respondents indicated a range (as given at the column head in the drove spreadsheet), midpoints of the ranges were used to provide a numerical figure for the analysis. For assays where more 10
098 these were used in the analysis. Where respondents indicated a range (as given at the column head in the drove spreadsheet), midpoints of the ranges were used to provide a numerical figure for the analysis. For assays where more 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 compounds were tested, a higher end value of a range could not exist determined and thus, the lower finish value of 'ten
000 compounds were tested, a higher end value of a range could not exist determined and thus, the lower finish value of 'ten![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000' was used (Table 4).
000' was used (Table 4).
Table iv Ranges given in information collection spreadsheet and midpoints used in analysis where needed. For assays which were tested more than x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times, the 'midpoint' of x
000 times, the 'midpoint' of x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was used
000 was used
Where companies provided data that did non encompass all of the 1980–2013 window, exclusion was considered to prevent any spurious impact on potential trends (run into results for details). In these cases, the utility scores and whatsoever comments given were included in the qualitative analyses.
Utility scores of '0' with accompanying reasons of 'did non exist at this time', 'not engaged in R&D requiring this' and 'do not believe in assay' were omitted during data analysis every bit these options do non reverberate the utility of the tests (just provided useful context to the data during assay).
Analysis of information
Raw information. Historical trends in the use of all in vitro tests reported were analysed by summing the full number of compounds tested in each year for all responding companies and presented graphically by yr. Trends for the use of individual in vitro tests such as mouse lymphoma assay or the Ames exam were analysed and presented in the same way. The relative use of in vitro assays in different fields was evaluated by comparing the total number of tests existence carried out in each field with the overall total tests existence carried out. In add-on, comparisons were made betwixt overall use and fields of use of in vitro tests in pharmaceutical companies and CROs from 1980 to 2013.
Normalised information. As well as computing raw data, additional information on overall historical trends in the apply of in vitro tests beyond all companies was revealed past normalising information within each company. This immune trends to exist viewed irrespective of the large variations in the total number of tests performed by individual companies. To do this, the total number of all in vitro tests carried out across all years (1980–2013), was calculated for each company. The total number of in vitro tests carried out in each individual yr was then divided past the total number of tests carried out by that visitor across all years and multiplied by 100 to give the percentage of tests carried out in each year. The mean per centum of in vitro tests carried out in each yr was then calculated across all companies. Historial trends in overall utilize of in vitro tests between CRO were revealed by normalising data for CROs and for Pharma. This allowed trends to be viewed irrespective of the large variation in the total number of tests performed by these two groups. To do this, the full number of all in vitro tests carried out beyond all years (1980–2013), was calculated for CROs and for Pharma; the full number of in vitro tests carried out in each individual year was and then divided by the total number of tests carried out past CRO or past Pharma across all years and multiplied by 100 to requite the percentage of tests carried out in each yr.
Utility scores
Utility scores were combined for each contributor to requite an boilerplate for each examination. Where no utility scores were recorded they were excluded from the final data analysis.
Results
Responses
Responses from seven companies (four pharmaceutical companies and three contract inquiry organisations (CROs)) were received in response to the ABPI survey. Some companies submitted data for multiple sites, including global locations, whereas others submitted data from UK sites only. Four of the seven respondents provided information in absolute values wheares three of the companies supplied data in ranges.
Five of the seven companies provided data across the total period of 1980 to 2013 although there were relatively low numbers of in vitro tests reported before 2005. One company provided information from 2005 onwards only – these data were included in the historical trends analysis since missing information from before 2005 for this i visitor had a minimal outcome on the analysis of historical trends confronting a background of low numbers of tests reported up to 2005 for the other five companies. In dissimilarity, one visitor provided data only from 2009 to 2013; responses from this visitor were non included in the analysis of historical employ of in vitro tests since data from other companies showed that many of the tests were in use before 2009 and inclusion of the 2009–2013 data from this i company would result in a larger credible increase from 2008 to 2009, thereby skewing the results. Although omitted from the historical trends analysis, information from this same company were used in analysis of breakup of in vitro tests co-ordinate to fields/disciplines where trends were non analysed historically. The scores and comments from this one company were likewise used in qualitative assay of data and to support interpretation and discussion of the overall trends.
Trend of utilize of in vitro tests
Fig. 1 displays the overall trend of utilize of in vitro tests between 1980 and 2013. Raw data (1A) showed a general year on twelvemonth increment including a large step upward from 2000 to 2005 but with a small downturn in 2011 and 2013. However, normalised data (1B) showing trends beyond all companies irrespective of the total number of tests carried out past each company showed a year by year increase and confirmed a strong upward trend in the use of in vitro tests between years 2000 and 2005.
 |
| | Fig. i Overall increase in the use of in vitro assays from 1980 to 2013. Bar graphs showing the tendency of use of in vitro tests by pharmaceutical manufacture (north = 6) from 1980 to 2013 generated using (A) raw data and (B) normalised information. | |
Breakdown of in vitro tests used past the pharmaceutical industry past fields/disciplines
Fig. 2 illustrates that in vitro tests in the fields of absorption, distribution, metabolism and excretion (ADME), condom pharmacology and genotoxicity account for 99.9% of the test carried out across the time period 1980–2013. Just a small proportion (0.i%) of the in vitro tests that were carried out was from other areas. A focused breakdown of this 0.1% revealed tests for dermal absorption, pare irritation, eye irritation and skin corrosion bookkeeping for the bulk with a few inhalation, endocrine disruption, development/reprotoxicology and phototoxicity tests also being reported. There was no reported use of in vitro techniques in the fields of carcinogenicity and immunotoxicology.
 |
| | Fig. ii Breakdown of in vitro tests used by the pharmaceutical industry (due north = 7) betwixt 1980 and 2013 past fields. The total number of individual tests done using in vitro tests in a item field is shown. DRE: development, reprotoxicology and endocrine disruption. | |
Comparisons betwixt use of in vitro tests by pharmaceutical companies and CROs
Fig. 3A shows the overall trends of apply of in vitro tests past pharmaceutical companies (top) and CROs (bottom) from 1980 to 2013. Overall, a large and steady increase in the use of in vitro tests was observed for both pharmaceutical companies and CROs since 2000. Of notation was the big deviation in total numbers of compounds between pharmaceutical companies and CROs with pharmaceutical companies peaking at >180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and CROs peaking at >500 tests per annum. In add-on, the employ of in vitro tests in pharmaceutical companies showed a slight drib in the utilize of in vitro tests between 2012 and 2013 in contrast to a marked increase in use for CROs in the same time menstruation.
000 and CROs peaking at >500 tests per annum. In add-on, the employ of in vitro tests in pharmaceutical companies showed a slight drib in the utilize of in vitro tests between 2012 and 2013 in contrast to a marked increase in use for CROs in the same time menstruation.
 |
| | Fig. iii Use of in vitro tests increased in both pharmaceutical companies and CROs from 1980 to 2013 only with a focus in different disciplines. (A) Total number of individual tests carried out by pharmaceutical companies (top; n = 3) and CROs (lesser; due north = 3) from 1980 to 2013 is shown; (B) Breakdown of apply of in vitro tests by discipline: Pie charts depict the relative use of in vitro test by pharmaceutical companies (meridian; northward = iii) and CROs (bottom; n = iii) according to disciplines. Data were presented every bit percentage values. | |
Fig. 3B illustrates the use of in vitro tests in pharmaceutical companies and CROs by subject. The pharmaceutical companies primarily used in vitro assays in iii fields: ADME, safe pharmacology and genotoxicity. In contrast, CROs carried out in vitro assays in various fields such as eye irritation, dermal assimilation and peel irritation. However, in mutual with data from the pharmaceutical companies, ADME also accounted for a large proportion (46%) of tests carried out by CROs.
Fig. four shows a comparison using normalised data betwixt the use of in vitro tests in the three largest disciplines (genotoxicity, safety pharmacology and ADME) by pharmaceutical companies and CROs between 1980 and 2013. CROs reported the earliest apply of in vitro tests in these disciplines with 0.5% of the overall CRO usage occurring in 1980 followed past the earliest upturn in use in the menstruum 2000–2008. The use of these tests past pharmaceutical companies was only axiomatic from 2000 only since so has shown a steady increment.
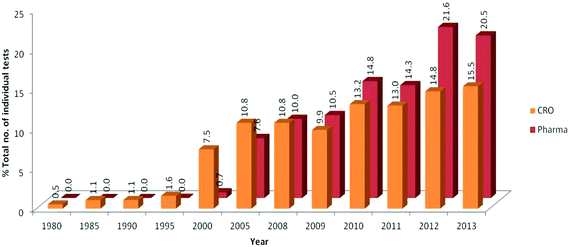 |
| | Fig. 4 Increasing trends of apply of in vitro tests in the three principal fields past pharmaceutical companies and CROs from 1980 to 2013. The three main areas of in vitro tests were genotoxicity, condom pharmacology and ADME. Data were contributed by three pharmaceutical companies and 3 CROs and was normalised. | |
Trend of utilise: in vitro tests from the three main disciplines
Fig. v shows the blueprint in historical uptake of tests inside the three principal disciplines of genotoxicity, safety pharmacology and ADME in the menstruation 1980 to 2013. For genotoxicity, utilise remained relatively low until 2010 but since then has continued to increase, with a particularly prominent increment in use of tests in this field from 2011–2012 (Fig. 5A). For prophylactic pharmacology, the use of in vitro assays has shown little increment, remaining at similar levels since the initial significant implementation effectually 2005 (Fig. 5B). For ADME, there was some usage as early as 1980 which remained depression but constant until a step change in 2000 followed by a steady increase (Fig. 5C).
 |
| | Fig. 5 Employ of in vitro tests in genotoxicity, prophylactic pharmacology and ADME increased differentially from 1980 to 2013. Console nautical chart shows historical trend of use of in vitro tests from the three selected fields: (A) genotoxicity, (B) safety pharmacology and (C) ADME from 1980 to 2013. Console on the left shows graphs generated using raw data whereas panel on the right shows graphs generated using normalised data. Number of companies that contributed data towards use of in vitro tests in each of the fields was shown at the top of each graph. | |
Trend of use: some examples of individual tests
Fig. half-dozen shows the historical trends of apply for two in vitro genotoxicity tests and 1 peel absorption test. DEREK and in silico tests in genotoxicity testing was noted in 1995 followed by a large increase to 2005 (Fig. 6A) and a slight increase in the use of these tests since this time. In contrast, since initial use in the mid-1990s, use of Ames II, Bluescreen and related assays (Fig. 6B) has increased only in an evidently inconsistent style.
 |
| | Fig. 6 Dissimilar trends in the use of individual in vitro tests in the field of study of genotoxicity and skin absorption past the pharmaceutical industry between 1980 and 2013. The historical trends of apply of (A) DEREK and other in silico projects (field: genotoxicity; northward = iv); (B) Ames II, MNU, Greenscreen and Bluescreen assays (field: genotoxicity; n = 4) and (C) in vitro/radio-analysis/LC-MS/MS (field: dermal absorption, n = two) are shown. | |
Fig. 6C shows the historical trends of use for in vitro tests of skin absorption. The usage of these tests has increased year on year since their initial introduction in 2000 except between 2008 and 2009 where usage remained abiding.
Utility
Fig. 7 shows the cess of the perceived utility for each of the individual in vitro tests in the survey. Overall, there was a loftier level of confidence (4: good but not exhaustive to 5: fantabulous) in the field of genotoxicity with a few notable exceptions; SOS UMU scored low on utility (2: not very predictive) and in silico Accomplish scored average (3: reasonable). All tests listed for pare irritation/absorption scored ≥4.nine whereas tests for ADME consistently scored between 3.viii and 4.four. The biggest variability within a field of study was seen in the utility scores for the tests for eye irritation where responses ranged from 1 (not predictive) for flourescein leakage to 4 (good only not exhaustive) for the BCOP.
 |
| | Fig. 7 Cess of utility for the individual in vitro tests averaged beyond all respondents in the areas of genotoxicity, condom pharmacology, skin irritation/corrosion/absorption, center irritation, inhalation, development/repro/endocrine toxicology, ADME and phototoxicity. Data were contributed by iv pharmaceutical companies and 3 CROs. Not all respondents offered scores on all tests; where tests or whole disciplines are absent-minded from the effigy, this is because no responses on utility for that examination were received. Numbers after confined depict number of data points contributing to the mean scores. | |
Discussion
In this study, nosotros sought to examine how the apply of in vitro techniques in the pharmaceutical manufacture has changed from 1980 to 2013. We also aimed to identify whether at that place has been a greater focus on developing in vitro techniques in some fields than others and compared the trends in use of in vitro assays between pharmaceutical companies and the CROs.
The data presented prove a large and continuing increase in the use of in vitro tests past the pharmaceutical manufacture. The slight plateau between 2012 and 2013 resolves when information are normalised such that each company'due south figures act as their ain control suggesting the apparent plateau is caused past a downturn in use by one or two big contributors to the survey. Overall, >20% of all in vitro tests reported were conducted in the last year of the survey window (2013) and >lxx% of all in vitro tests reported were conducted since 2010. This increase is encouraging in the context of the 3Rs, a gear up of principles that outlines the replacement, reduction and refinement of the use of animals in research. 3 Indeed, expenditure on research and development past the pharmaceutical industry has grown past £i billion since 2002 16 yet the Home Function figures for animal use in the UK 17 by commercial organisations have stayed largely unchanged with around 1–1.5 meg procedures a year reported since 1995. 17 This increased investment in UK R&D in the absence of an substantial increase in brute use could be attributed at least in office to the increased employ of in vitro tests described here. It is still worth noting that certain approaches with loftier reported utilize herein such as DEREK offer new technological possibilities rather than replacing existing in vivo assays per se. Nonetheless, such in silico approaches could exist viewed as helping to select and prioritise compounds for development with a amend profile and hence probability of success.
The comparisons of in vitro work carried out by CROs with that carried out by pharmaceutical companies reveal interesting trends. For example, CROs conducted only 0.25% of the tests reported in the period 1980 to 2013 reflecting their relatively smaller size but nonetheless conducted a much wider range of in vitro tests when compared with pharmaceutical companies. For instance, 99.9% of the reported in vitro assays conducted by pharmaceutical companies were in the iii fields of ADME, prophylactic pharmacology and genotoxicity peradventure reflecting a trend in the manufacture to focus resource in certain higher throughout areas and outsource to CROs tests carried out less frequently. In contrast, although CROs did conduct work in the three areas of ADME, safe pharmacology and genotoxicity, 44% of the in vitro tests at CROs were in diverse fields such equally eye irritation, dermal absorption and pare irritation. In dissimilarity, <0.01% of all in vitro tests washed past the pharmaceutical companies was from disciplines other than the top three. A farther assay of trends over fourth dimension in these three chief areas (genotoxicity, safe pharmacology and ADME) suggests that CROs were engaged in conducting in vitro tests in 1 or more than of these fields before than pharmaceutical companies, with some use reported since the 1980s.
Comparisons of trends over time in the three main areas reported (genotoxicity, safety pharmacology and ADME) suggests differences in patterns of uptake. There was a tedious but steady increment in the use of genotoxicity tests since 1980 whereas there was a step change in reported use of both genotoxicity and prophylactic pharmacology assays since 2005. Interestingly, there was a pocket-sized simply steady employ of ADME from the start of the survey in 1980 with a steady increase thereafter. Although the reasons behind these uptake trends are likely to be multifactorial, introduction of new and modifications of existing International Committee on Harmonisation (ICH) guidelines may explain some of the data. For example, ICH S2A (Regulatory Genotoxicity Tests for Pharmaceuticals) was finalised in 1995 followed in 1997 by ICH S2B 18 which outlines the standard battery for genotoxicity testing and provides recommendations on the evaluation of examination results. Together, these two guidelines are probable to explain some uptake in 1995–2000, the small stride seen in 2005 and the subsequent steady ascent in the use of in silico and in vitro tests for genotoxicity testing for pharmaceuticals. In this context, information technology'southward worth noting that ongoing ICH revisions and their adoption often tend to be driven by enquiry into and validation of in vitro alternatives conducted by and published in collaboration between pharmaceutical companies, CROs and academia.
Regarding safety pharmacology, ICH S7A which addresses definition, objectives and scope of safe pharmacology studies for pharmaceuticals was finalised in 2000. 18 Only 44 tests were reported for safety pharmacology up to and including 2000 but then >28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tests were reported in 2005 lone with a steady level and slight rise thereafter.
000 tests were reported in 2005 lone with a steady level and slight rise thereafter.
The earlier uptake of tests for ADME could be explained by the earlier adoption of a guideline in this expanse; S3A (Guidance on Toxicokinetics) reached step iv (finalization) in 1994; 18 the data presented here suggest some lag in uptake with only 12 tests reported in 1990 and in 1995 simply then a steady increase to >6300 tests per annum from 2000 onwards. Interestingly both ICH S7A and ICH S3A largely depict in vivo tests rather than in vitro alternatives, but it could be argued that clarity on acceptable biological endpoints can provide a base of operations for developing in vitro alternatives irrespective of the origin of the endpoints.
A focused assay of 2 of the clusters of in vitro genotoxicity tests (DEREK/other in silico tests versus Ames 2/MNU/Greenscreen/Bluescreen) revealed very dissimilar trends in uptake. 'DEREK/other in silico tests' showed a rapid uptake between 2000 and 2005 with petty farther increase; this suggests that these test may already have been used to maximum effect since their introduction, or have not been developed further. Some other explanation is the relatively low utility score returned on DEREK with responses between 'reasonable' and 'good' compared with some of the other tests in the genotoxicity battery that were rated as good or first-class.
Since initial uptake in the mid-1990s, the use of Ames Ii/MNU/Bluescreen/Greenscreen related assays has increased only inconsistently. The utilize of MS in testing pare absorption has also increased steadily since its initial introduction effectually 2000. This is likely to be a reflection of steadily increasing demand coupled with increasing accessibility and an overall high score in utility; in that location accept been incremental improvements in the accessibility and reliability of the technologies required for this assay year past year.
The arroyo taken in this paper has helped to quantify the uptake of in vitro tests over a flow of 33 years. The approach was never intended to be exhaustive since information technology depends upon companies retrieving historical data; indeed information technology is likely that many other tests were carried out that could not be accounted for, specially at the earlier time points. Areas such as reprotoxicology likewise appear to be underrepresented in the data ready for similar reasons. Thus the information fix nearly probable under-report the actual number of tests conducted but withal offer encouraging insight on the upward trends in in vitro alternatives to in vivo testing to complement the regulatory requirements for the prophylactic evaluation of candidate drugs earlier clinical trials.
Overall, the survey and approach used to quantify employ of in vitro tests confirm our recent conclusions drawn from published literature 13 that the pharmaceutical industry has a strong commitment to the development and uptake of in vitro test methods and has seen significant success in fundamental areas such as genetic toxicology, peel absorption and reproductive toxicology. 19 Indeed, many of the on-going in vitro initiatives to seek and formally validate culling and in vitro tests are focused on pharmaceuticals as illustrated by Chapman et al. xix Developments in validation and regulatory guidance effectually in vitro techniques accept as well facilitated uptake and adoption – this is notable in the timing of uptake connected to the introduction of new guidance such every bit that from ICH. However, continued uptake of in vitro alternatives depends on reliable and relevant models and there is still much to achieve in this expanse. This written report has highlighted the need for further investment in the development of in vitro tests in particular fields, such as immunotoxicity, every bit well as a demand to proceed refining assays that are currently used. The data suggest that implementation can also be a challenge; some tests with high scores on utility took many years to implement or are only used in a few companies. Many of the in vitro assays that have been adult in areas such every bit genetic toxicology and electrophysiology score low on utility – this could exist due to a high level of fake positive results which makes extrapolation to the homo situation difficult. In add-on, the reliability of in silico testing to predict condom signals remains in its infancy and has even been chosen into question in a recent paper from Cook et al. (2014). 20
Many of the tests reported in this report reduce and refine fauna employ by allowing early loftier throughput screening of compounds, reducing the number of ineffective or unsafe compounds progressing to in vivo studies. On the other hand, others are straight alternatives to in vivo methods. I instance is the EpiSkin examination that uses reconstructed human epidermis as a replacement for the rabbit skin irritation tests, and has been validated and endorsed past organizations including ECVAM. 21 The first use of the EpiSkin alternative reported in this report was in 2008 with connected increment in utilise from 2008–2013, reflecting its validation by ECVAM in 2007. Another encouraging example is the uptake by manufacture since 2008 of the Bovine Corneal Opacity and Permeability (BCOP) test, an in vitro test developed as an alternative to the rabbit Draize eye irritation exam. The BCOP assay uses excised creature tissue to supercede the in vivo studies, and when used in combination with other in vitro tests, could fully replace the utilise of an animal model in the future. 22
In summary, the data show a large increment and a continuing up trend in development and adoption of in vitro alternatives to fauna testing in pharmaceutical drug development providing new opportunities to improve success rates coupled with a strong commitment to the 3Rs. Withal despite the encouraging trend at that place is still much to be washed; there is a pressing need to ameliorate success rates in the pharmaceutical industry and also to brand failure less costly perchance via the development and validation of farther in silico and in vitro laboratory tests that could address the chief reasons for failure: unexpected toxicity and/or lack of efficacy. twenty Collaboration across industry, CROs, academia and authorities 23 will be key to time to come success via identifying and exploiting the best knowledge and expertise.
References
- ICH Guideline M3(R2), Guidance on Nonclinical Safety Studies for the Conduct of Homo Clinical Trials and Marketing Authorization for Pharmaceuticals, 2009, http://www.ich.org/products/guidelines/multidisciplinary/article/multidisciplinary-guidelines.html.
- ICH Guideline S9, Nonclinical Evaluation for Anticancer Pharmaceuticals, 2009, http://www.ich.org/products/guidelines/safety/article/safety-guidelines.html.
- West. M. S. Russell and R. L. Burch, The Principles of Humane Experimental Technique, Methuen, London, 1959 Search PubMed.
- Animals (Scientific Procedures) Human activity 1986.
- European union Directive 2010 on the protection of animals used for scientific purposes. Directive 2010/63/EU.
- EMA Revised Concept newspaper on the need for revision of the position on the replacement of beast studies by in vitro models (CPMP/SWP/728/95) CPMP/SWP/728/95), 2012, http://world wide web.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500130365.pdf.
- NC3Rs, 2014, http://www.nc3rs.org.great britain/about-usa [accessed 18 th October 2014].
- Home Function, Department for Concern Innovation & Skills and Department of Health, Working to reduce the use of animals in scientific research, 2014, https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/277942/bis-xiv-589-working-to-reduce-the-apply-of_animals-in-research.pdf [accessed 18th October 2014].
- Southward. Ward, Applicability domain of BCOP and ICE extended to identifying ocular non-irritants, 2013, http://alttox.org/applicability-domain-of-bcop-and-ice-extended-to-identifying-ocular-non-irritants-two/ [accessed 18th October 2014].
- NC3Rs, Working with the pharmaceutical industry: pioneering better science 2004–2014, NC3Rs, London, 2014, pp. one–31 Search PubMed.
- Understanding Animate being Research, Concordat on openness on animal research in the Uk, Understanding Animal Research, London, 2014, pp. 1–10 Search PubMed.
- EFPIA, Putting animal welfare principles and 3Rs into action: European pharmaceutical industry 2011 report, EFPIA, Brussels, 2012, pp. one–21 Search PubMed.
- EFPIA, Putting animate being welfare principles and 3Rs into action: European pharmaceutical industry 2012 update, EFPIA, Brussels, 2013, pp. 1–15 Search PubMed.
- S. Cunningham, N. Partridge and R. Roberts, Quantifying the pharmaceutical industry's contribution to published 3Rs research 2002–2012. Manuscript draft, Elsevier Editorial System (tm) for Toxicology Reports, 2014.
- ABPI Expert Networks, http://www.abpi.org.uk/about-the states/how-we-work/Pages/working-members.aspx.
- ABPI, U.k. Pharmaceutical R&D Expenditure 2000–2012, 2012. http://world wide web.abpi.org.britain/manufacture-info/knowledge-hub/randd/Pages/expenditure.aspx#half-dozen.
- Home Office, Statistics of scientific procedures on living animals, United kingdom of great britain and northern ireland 2013, 2013, https://www.gov.uk/government/statistics/statistics-of-scientific-procedures-on-living-animals-neat-united kingdom-2013.
- ICH, 2014http://www.ich.org/products/guidelines/safety/article/safety-guidelines.html.
- K. Chapman, H. Holzgrefe, L. Black, M. Dark-brown, G. Chellman, C. Copeman, J. Couch, S. Creton, South. Gehen, A. Hoberman, L. Kinter, South. Madden, C. Mattis, H. Stemple and Due south. Wilson, Reg. Tox. Pharm., 2013, 66, 88–103 CrossRef CAS PubMed.
- D. Melt, D. Dark-brown, R. Alexander, R. March, P. Morgan, K. Satherwaite and M. Panagalos, Nat. Rev. Drug Discovery, 2014, xiii, 419–431 CrossRef CAS PubMed.
- H. Spielmann, et al. The ECVAM international validation written report on in vitro tests for acute skin irritation: study on the validity of the EPISKIN and EpiDerm assays and on the Peel Integrity Office Test, Altern. Lab. Anim., 2007, 35, 559–601 CAS.
- OECD, 2009. OECD Guidelines for Testing of Chemicals; Test Guideline 437; Bovine Corneal Opacity and Permeability Test Method for Identifying Ocular Corrosives and Astringent Irritants.
- J. Hunter, Drug Discovery World, Spring, 2014, nine–15 Search PubMed.
Footnote |
| † Now at ApconiX, BioHub at Alderley Park, SK10 4TG. |
|
| This journal is © The Royal Society of Chemical science 2015 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 information points beyond all years and all assays. Overall, there was a steady increase in the use of in vitro tests by the pharmaceutical industry between 1980 and 2013; indeed >20% of all in vitro tests reported were conducted in the last yr of the survey window (2013) and >seventy% of all in vitro tests reported were conducted since 2010. Use of in vitro tests peaked at >190
000 information points beyond all years and all assays. Overall, there was a steady increase in the use of in vitro tests by the pharmaceutical industry between 1980 and 2013; indeed >20% of all in vitro tests reported were conducted in the last yr of the survey window (2013) and >seventy% of all in vitro tests reported were conducted since 2010. Use of in vitro tests peaked at >190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tests per annum in 2012; >99% of this usage was in the three main areas reported of ADME, condom pharmacology and genotoxicity. Trends and pace changes in uptake were virtually notable in the iii main areas of ADME, safety pharmacology and genotoxicity and may be explained by the timing of adoption of the relevant International Committee on Harmonisation (ICH) guidelines. Trends in uptake may as well exist explained by perceptions of utility where scores varied from poor (Middle Irritation – flourescein leakage) to splendid (Genotoxicity – Ames and Peel irritation – EpiSkin/Epiderm). In summary, the data show a large increase and a standing upwards trend in development and adoption of in vitro alternatives to animal testing in pharmaceutical drug development providing new opportunities to improve success rates coupled with a potent delivery to the 3Rs.
000 tests per annum in 2012; >99% of this usage was in the three main areas reported of ADME, condom pharmacology and genotoxicity. Trends and pace changes in uptake were virtually notable in the iii main areas of ADME, safety pharmacology and genotoxicity and may be explained by the timing of adoption of the relevant International Committee on Harmonisation (ICH) guidelines. Trends in uptake may as well exist explained by perceptions of utility where scores varied from poor (Middle Irritation – flourescein leakage) to splendid (Genotoxicity – Ames and Peel irritation – EpiSkin/Epiderm). In summary, the data show a large increase and a standing upwards trend in development and adoption of in vitro alternatives to animal testing in pharmaceutical drug development providing new opportunities to improve success rates coupled with a potent delivery to the 3Rs.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 098 these were used in the analysis. Where respondents indicated a range (as given at the column head in the drove spreadsheet), midpoints of the ranges were used to provide a numerical figure for the analysis. For assays where more 10
098 these were used in the analysis. Where respondents indicated a range (as given at the column head in the drove spreadsheet), midpoints of the ranges were used to provide a numerical figure for the analysis. For assays where more 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 compounds were tested, a higher end value of a range could not exist determined and thus, the lower finish value of 'ten
000 compounds were tested, a higher end value of a range could not exist determined and thus, the lower finish value of 'ten![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000' was used (Table 4).
000' was used (Table 4).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times, the 'midpoint' of x
000 times, the 'midpoint' of x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was used
000 was used 

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and CROs peaking at >500 tests per annum. In add-on, the employ of in vitro tests in pharmaceutical companies showed a slight drib in the utilize of in vitro tests between 2012 and 2013 in contrast to a marked increase in use for CROs in the same time menstruation.
000 and CROs peaking at >500 tests per annum. In add-on, the employ of in vitro tests in pharmaceutical companies showed a slight drib in the utilize of in vitro tests between 2012 and 2013 in contrast to a marked increase in use for CROs in the same time menstruation. 




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tests were reported in 2005 lone with a steady level and slight rise thereafter.
000 tests were reported in 2005 lone with a steady level and slight rise thereafter.
0 Response to "What Is In Vitro Testing Vs Animal Testing"
Post a Comment